Catalytic Properties of Different Types of Active Sites in Zirconosilicate Zeolites Distinguished for the First Time
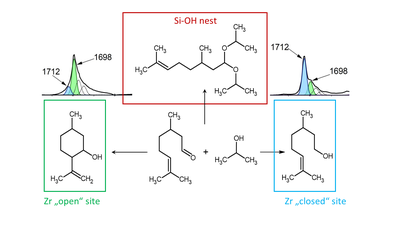
Jan Přech, together with postdoctoral researcher Kinga Gołabek and his students from the Heterogeneous Catalysis and Advanced Materials Group (KFMCh), has published an article in the prestigious journal ACS Catalysis. The work presents the very first observation of a correlation between the distribution of different types of active sites in zirconosilicate zeolites and the selectivity of the competing chemical reactions they catalyze. In other words, it demonstrates that each type of active site (several of which can be found in zirconosilicates and which differ primarily in their acid strength) selectively catalyzes a different chemical reaction, if the reaction system allows it.
Distinguishing between different types of active sites in zirconosilicate zeolites is very challenging, as no direct spectroscopic technique exists to enable such differentiation. In addition to observing the correlation between catalyst structure and activity, the published article also introduces a relatively simple spectroscopic method for distinguishing different active sites using a molecular probe observable at laboratory temperature.
ACS Catalysis publishes original research articles in homogeneous, heterogeneous, and enzymatic catalysis and has an impact factor of 13.3 (2024), the third highest among purely catalysis-focused journals, although catalysis is not indexed as a separate discipline in scientific databases.
Zeolites are important industrial catalysts, ion exchangers, and adsorbents used in hydrocarbon processing, gas purification and separation, water treatment, and even agriculture. In most of these applications, their acidic properties—generated by the presence of aluminum in an otherwise siliceous framework—are utilized in combination with their precisely defined porous crystalline structure.
Zirconosilicate zeolites are water-tolerant Lewis acids that catalyze redox and isomerization reactions (especially hydrogen transfer reactions, dehydration, sugar isomerization, etc.), which are gaining increasing importance in efforts to reduce industrial waste and to implement bio-based feedstocks. Together with tin-silicate and hafnium-silicate zeolites, they belong to a class of materials discovered after 2000 whose full potential has not yet been explored.
The Heterogeneous Catalysis and Advanced Materials Group has been engaged in long-term research of these materials, for example within prestigious ERC-CZ grant awarded to Assoc. Prof. Mariya Shamzhy and the EXPRO project of Prof. Jiří Čejka. The mentioned article is a piece of work carried out entirely at our faculty.